The clinical study evaluates use of the investigational therapy, BBI-355, in patients with ovarian or endometrial cancer that has gene amplification (also known as copy number gain), such as CCNE1, MYC, or KRAS.

A clinical study that could be right for you
The POTENTIATE study is a clinical trial for patients with ovarian or endometrial cancer that has gene amplification (also called copy number gain). The study is testing the safety and tolerability of a non-chemotherapy, investigational therapy in the form of an Oral Pill. The POTENTIATE study is now enrolling patients.
Additional information can be obtained from the national clinical study registry, Clinicaltrials.gov (click here NCT05827614).
Why should I participate in the POTENTIATE Trial?
- The investigational therapy, BBI-355, is an Oral Pill that you take conveniently at home.
- BBI-355 has been shown to have anti-tumor activity in gene amplified ovarian and endometrial experimental cancer models.
Have your cancer doctor (or oncologist) explain your eligibility and the potential benefits and risks to you before considering joining the POTENTIATE trial.

See if you are Eligible
Women who have been diagnosed with Endometrial Cancer or Ovarian Cancer that has gene amplification (or copy number gain), such as CCNE1, MYC, or KRAS
Other treatments are not working for you
(e.g., chemotherapy)
If you have a next-generation sequencing (NGS) Report - This would be a PLUS
Where is the Potentiate Trial Taking Place?
Check the locations below to see if there is a trial location close to you.
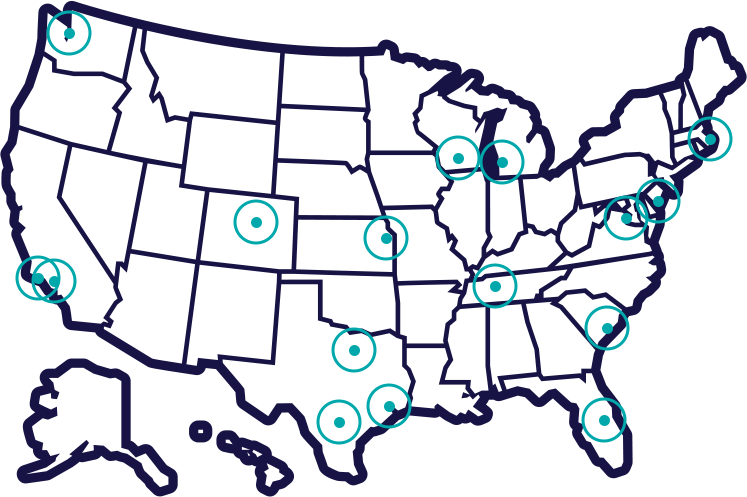
California Locations:
UCLA Medical Center
2825 Santa Monica Boulevard, Suite 600, Santa Monica, CA 90404
Lisa Yonemoto
(310) 633-8400 x16045
Sarcoma Oncology Research Center
2811 Wilshire Blvd., Suite 414, Santa Monica, CA 90403
Victoria Chua
(310) 552-9999
Colorado Locations:
Sarah Cannon Research Institute at HealthONE
1800 Williams Street, #300, Denver, CO 80218
(720) 754-2610
Florida Locations:
Sarah Cannon Research Institute at Florida Cancer Specialists
805 Currency Circle, Lake Mary, FL 32746
Lacey Gebben
Kansas Locations:
University of Kansas
2330 Shawnee Mission Pkwy, MS 5003, Westwood, KS 66205
Nurse Navigators
(913) 945-7552
Massachusetts Locations:
Dana Farber Cancer Institute
330 Brookline Ave,
Boston, MA 02215
Jasmine Matsumoto
(617) 632-6297
Beth Israel Deaconess Medical Center
330 Brookline Ave,
Boston, MA 02215
Victoria Weden
617-975-7489
Michigan Locations:
South Texas Accelerated Research Therapeutics (START) Midwest
5800 Foremost Drive,
SE Suite 100,
Grand Rapids, MI 49546
Julie Burns
New York Locations:
Memorial Sloan Kettering Cancer Center
1275 York Ave.,
New York, NY 10065
South Carolina Locations:
Medical University of South Carolina
30 Courtenay Drive, MCS 772 Rm 117, Charleston, SC 29425
Carly Fecio
Tennessee Locations:
Sarah Cannon Research Institute Oncology Partners
1100 Dr. MLK Jr. Blvd., Suite 800, Nashville, TN 37203
Brittany Callaway
Texas Locations:
MD Anderson Cancer Center
1515 Holcombe Blvd., Unit 455, Houston, TX 77030
Jessica Rhudy
NEXT Oncology Dallas
6750 North MacArthur Blvd, Suite 250, Irving, TX 75039
Patient Navigators
(737) 610-5202
Virginia Locations:
NEXT Oncology Virginia
8613 Lee Highway
Fairfax, VA 22031
Carrie Friedman
Clinical Trial Navigator
(703) 636-1473
Washington Locations:
University University of Washington
825 Eastlake Avenue East,
Seattle, WA 98109
Harini Ramachandran
(206) 606-6448
Wisconsin Locations:
University of Wisconsin
600 Highland Avenue,
K4/518 Clinical Science Center, Madison, WI 53792
CancerConnect
(800) 622-8922
Frequently Asked Questions
What is a clinical study?
Clinical research studies, or clinical trials, help scientists and doctors evaluate the safety and potential anti-cancer activity of new medical treatments. The study may take place at a hospital, clinic, university, or doctor’s office.
Who is conducting the trial?
Boundless Bio, a biopharmaceutical company, is sponsoring the study, which is being conducted by cancer doctors (or oncologists) at the clinical study sites.
What is an investigational therapy?
Investigational means that the therapy has not been approved for medical use and is currently being studied to understand its safety and potential anti-cancer activity. Studies of investigational therapies may only be performed with the permission of, and under the oversight of, the regulatory authority of the country where the study is being conducted, such as the US Food and Drug Administration (FDA) in the United States or the European Medicines Agency (EMA) in Europe. BBI-355 is an investigational therapy not yet approved by the FDA or EMA.
How do I know if I have ovarian or endometrial cancer that has gene amplification (e.g., CCNE1, MYC, KRAS, or other gene)?
This type of information about your cancer is called genomic information. To understand the genomic information about your cancer, a sample of your tumor, or sometimes a sample of your blood, will be tested for the presence of CCNE1, MYC, KRAS, and other genomic biomarkers. This type of testing is called next-generation sequencing, or ‘NGS’ testing. Ask your cancer doctor about your next-generation sequencing (NGS) report. NGS testing and the resulting NGS report may have been done by any of these, or other, testing companies – Foundation Medicine, Tempus, Guardant, or Caris, and will contain genomic information about your cancer, including whether you have CCNE1, MYC, KRAS, or another gene amplified in your cancer.
Once I have my NGS testing report, how can I tell if I have an amplification – like CCNE1, MYC, KRAS or others?
Typically, on the first or second page of the report you will see a list of genes in “Genomic Findings” or “Genomic Variants” or “Molecular Findings”. On this list, you may see genes with “amplification” or “copy number gain”. If your report includes CCNE1, MYC, KRAS, or other genes with amplification or copy number gain, you may be eligible for the study.
Click here to see where to look on your NGS report >>
If I don’t have an NGS report, how can I obtain one?
Speak to your cancer doctor about genomic testing of your cancer.
What will happen during the study?
The oncology care team at the clinical study site will explain in detail to you what participation in the POTENTIATE study means for you. Participation is completely voluntary, and you are permitted to change your mind about participation at any time.
People choosing to participate will be asked to do the following:
• Attend visits and have assessments such as blood draws to find out if you qualify for the study.
• Take the investigational therapy as directed by the study doctor.
• Participate in follow up appointments including additional blood draws and tumor imaging (scans).
How long will I be in this study?
The length of time you will be in the study depends on how your body responds to the therapy. You will always have the option to stop at any time.
Who should I contact about joining the POTENTIATE study?
You or your cancer doctor can contact a study Investigator or the study coordinator at any of the clinical study sites participating in the conduct of the POTENTIATE study.
What if I don’t see a location near me?
Your primary care physician or cancer doctor may be able to refer you to one of the clinical study sites. Travel support may be available to help you travel to one of the clinical study sites.
Additional sites may be opening, so keep checking back for updates.
Ready to learn more about how to join the study? Click the button below and reach out today.




